Which of the Following Intermolecular Forces Is the Strongest Apex
The strongest intermolecular forces is hydrogen bond. Question 37 Which of the following substances has the strongest intermolecular forces.
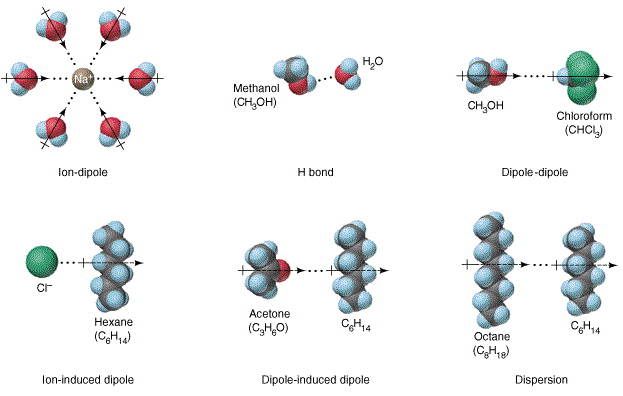
4 2 Intermolecular Forces Chemistry Libretexts
London dispersion dipole-dipole H-bonding.

. Induced dipoles are the weakest attractive forces and last for a short period of time. The strongest intermolecular forces are in ion-ion bonds which happen when a metal bonds to another metal. Which of the following orders is correct from weakest to strongest for the strength of intermolecular forces in neutral molecules on a per interaction basis.
Which is the strongest intermolecular force below. What is the strongest intermolecular force present for each of the following molecules. 3Van der waals forces temporary dipole-induced dipole- weakest.
QUESTION 3 Which of the following intermolecular forces of attraction is the strongest. Solutions What is the strongest intermolecular force present for each of the following compounds. Among cellulose poly vinyl chloride nylon and natural rubber the polymer in which the intermolecular force of attraction is weakest is asked Sep 21 2020 in Chemistry by Punamsingh 960k points.
List the substances BaCl2 H2 CO HF and Ne in order of increasing boiling points. However the London Dispersion Forces in CS2 are so strong that they overpower the strength of both the LDFs and the dipole-dipole forces in COS. These are strong bonds compared to ionic and hydrogen bonds.
CS2 and COS both have London Dispersion Forces but since COS is a polar molecule it also exhibits dipole-dipole forces. Types of Intermolecular Forces. They are the strongest type of intermolecular force that can act on polar and nonpolar molecules.
South Dade Senior High School. Sometimes a compound has more than one intermolecular force. The unit cell for sodium chloride shows ordered closely-packed ions.
The next strongest forces are ion-dipole bonds which happen when metals bond to nonmetals. They are the primary intermolecular attractive forces that act between nonpolar molecules. 1 hydrogen H 2 London dispersion forces 2 carbon monoxide CO London dispersion forces 3 silicon tetrafluoride SiF 4 London dispersion forces 4 nitrogen tribromide NBr 3 dipole-dipole forces 5 water H 2 O hydrogen bonding 6 acetone CH 2.
The third strongest force is a type of dipole-dipole force called hydrogen bonding. For example water has London dispersion dipole-dipole and hydrogen bonds. Therefore CS2 has a higher boiling point.
B Hydrogen bonding is the strongest intermolecular attractive force and is listed first. Textbook Solutions Expert Tutors Earn. Dipole-dipole forces c- dispersion forces d London forces e dipole-induced dipole forces QUESTION 4.
They determine how strong dipole moments will be in polar covalent molecules. The three most common intermolecular forces from strongest to weakest are. Chemistry questions and answers.
D london dispersion. Types of Intermolecular Forces What is the strongest intermolecular force present for each of the. The types of molecules with the strongest intermolecular forces are those molecules held together by covalent bonds.
The boiling point of a substance is proportional to the strength of its. The attractive forces are stronger for ionic substances than for molecular ones The intermolecular forces of the remaining substances depend on molecular weight polarity and hydrogen bonding. A hydrogen bonding b.
Which type of intermolecular force arises from permanent partial charges caused by the electronegativity differences among atoms. They are the strongest type of intramolecular force that can act between atoms. An intermolecular force is an attractive force that arises between the positive components or protons of one molecule and the negative components or electrons of another molecule.
London dipole-dipole hydrogen bonding Which of the following intermolecular forces is the strongest on a per interaction basis. Which of the following intermolecular forces is available to all molecules. Dipole - dipole which includes hydrogen bonds dipole - induced dipole London dispersion forces Intermolecular forces in methanol The strongest intermolecular forces in methanol are hydrogen bonds an especially strong type of dipole-dipole interaction.
These are weekest of all the intermcolecular forces and are between the electrons of one molecule atom and nucleus of another molecules. An organic molecule that can hydrogen bond to water will be ________ soluble than a similarly sized molecule. O CH3-CH2-CH3 OCH3-O-CH3 OCH3-CH2-C1 O CH3-CH2-OH OCH3-CH2-SH 25pte The boiling point of H2O is higher than H2S H2Se and HyTe.
Various physical and chemical properties of a substance are dependent on this force. Strength of intermolecular forces listed from weakest to strongest. Which of the following intermolecula attractions is responsible for the high boiling point of.

Practice Docx 1 1 7 Practice Intermolecular Forces And The Properties Of Solids And Liquids Ap Chemistry Sem 2 Name Kayla James Points Course Hero

What Makes A Good Nucleophile Master Organic Chemistry Organic Chemistry Chemistry Physics And Mathematics
3 2 Intermolecular Forces Origins In Molecular Structure Chemistry Libretexts

Types Of Intermolecular Forces 3rd Grade Math Worksheets Fraction Word Problems Free Math Worksheets

Order The Molecules From The Strongest Intermolecular Force Top To The Weakest Bottom Refer The Brainly Com
Intermolecular Force Chemistry Organic Chemistry

Which Of The Following Is Strongest Type Of Interaction That Occurs Between The Atom Within The Brainly Com

Physical Organic Chemistry Prepared By Dr Khalid Ahmad Shadid Islamic University In Madinah Department Of Chemistry Ppt Download

Which Type Of Force Is The Weakest Brainly Com

Chem 116 Pogil Week03 1 Pdf Chem 116 Pogil Worksheet Week 3 Intermolecular Forces Liquids Solids And Solutions Why Most Substances Can Exist In Course Hero

1aa3 Notes On Intermolecular Forces

Which Intermolecular Force Would Affect The Boiling Point The Least Brainly Com
What Is Meant By Non Directional Forces Of Attraction Quora

What Forces Hold Molecular Solids Together Lisbdnet Com

Types Of Intermolecular Forces 3rd Grade Math Worksheets Fraction Word Problems Free Math Worksheets
Comments
Post a Comment